A new medication was approved in August 2023 by the United States Food and Drug Administration (FDA). The medication is zuranolone (brand name Zurzuvae™).
Zuranolone is the first oral medication that is specifically FDA-approved for the treatment of postpartum depression.
What’s the big deal?
1. We have been treating postpartum depression with oral medication all along but until now, none of them had a specific FDA indication for the treatment of postpartum depression.
Note: Brexanolone (brand name Zulresso®) was previously approved by the FDA for the treatment of postpartum depression but it has to be given by intravenous infusion.
2. Zuranolone is completely different in its pharmacological effect from currently available oral antidepressants (SSRIs, SNRIs, bupropion, and so on). Like brexanolone, it is a “neuroactive steroid gamma-aminobutyric acid (GABA) A receptor positive modulator“.
3. It can have a rapid onset of effect in at least a subset of patients which can be particularly helpful in postpartum depression, allowing the mother to resume interaction with her newborn (and other children, if any).
4. It is intended to be used for only 14 days.
5. It can be taken alone OR along with another oral antidepressant.
FDA-indication
Zuranolone is indicated for the treatment of postpartum depression (PPD) in adults.
Mechanism of Action/ Pharmacodynamics
Zuranolone is a “neuroactive steroid gamma-aminobutyric acid (GABA) A receptor positive modulator“.
Let’s explain the terminology involved in the preceding statement.
– Gamma-aminobutyric acid (GABA) is the main inhibitory neurotransmitter in the adult central nervous system (Allen et al., 2023).
– GABA receptors —that is, receptors at which the neurotransmitter binds — are of two different types and are called GABAA and GABAB (Allen et al., 2023).
– “Steroid” refers to substances that have a certain chemical structure. This is similar to some substances being called “monoamines”.
– “Neuroactive steroid”(NAS) simply refer to those steroids, natural or synthetic, that have effects on the central nervous system.
– The term “modulator” is used to indicate that the substance is not by itself either an agonist or an antagonist; instead, it “modulates” the response of the receptor to other substances that activate the receptor.
– And, lastly, the term “positive” modulator means that when it modulates the response at the receptor, it increases the response rather than decreases it.
Contraindications
None
Warnings and Precautions
Zuranolone carries a boxed warning (often wrongly called a “black box” warning) regarding driving impairment due to central nervous system depressant effects. See the image below: 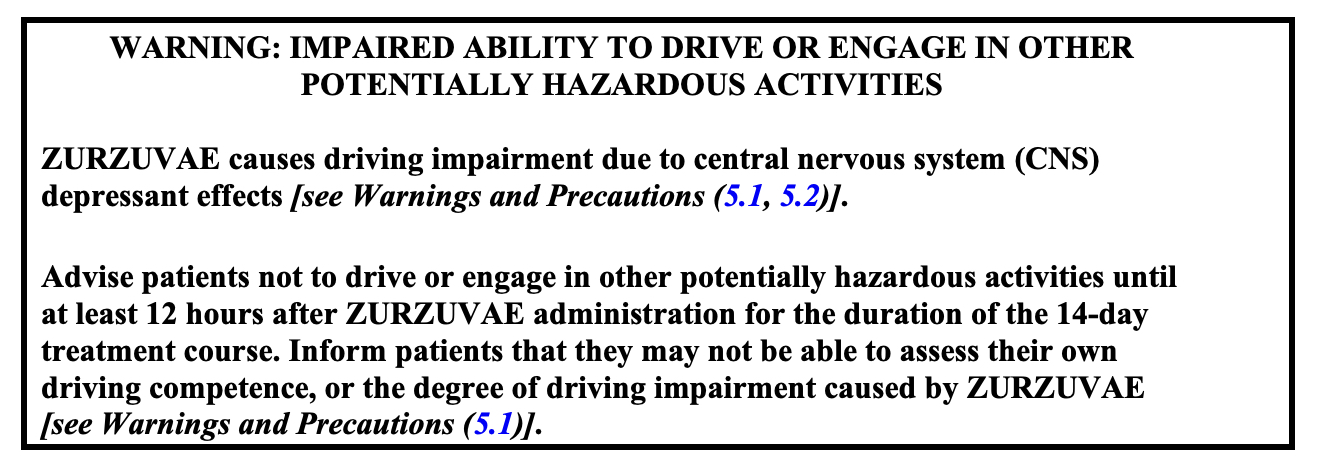 Note: Brexanolone (Zulresso®), which has the same mechanism of action as zuranolone, is only available through a special REMS program because there is a concern that excessive sedation or sudden loss of consciousness can occur while the intravenous injection of brexanolone is being given.
Note: Brexanolone (Zulresso®), which has the same mechanism of action as zuranolone, is only available through a special REMS program because there is a concern that excessive sedation or sudden loss of consciousness can occur while the intravenous injection of brexanolone is being given.
For other Warnings and Precautions, please see the full Prescribing Information. They relate to:
– Suicidal thoughts and behavior
– Embryo-fetal toxicity
Dosage and Administration
– The recommended dose is 50 mg/day, once daily, in the evening, for 14 days. At least for now, it is not FDA-approved for use for more than 14 days.
– It should be taken with food that contains fat. How much food? About 400 to 1000 calories. How much fat? About 25% to 50% of the calories.
– It can be taken alone OR along with another oral antidepressant.
Dosage forms and strengths
Capsules: 20 mg, 25 mg, and 30 mg
Important! This page does not provide all the information needed to prescribe this medication. Please refer to the full Prescribing Information (see link below) before prescribing this medication.
Related Pages
Brexanolone (brand name Zulresso®): Basic information
Treatments: Main menu (index and links)
References
Zurzuvae™ Prescribing Information
Allen MJ, Sabir S, Sharma S. GABA Receptor. 2023 Feb 13. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan–. PMID: 30252380.
Althaus AL, Ackley MA, Belfort GM, Gee SM, Dai J, Nguyen DP, Kazdoba TM, Modgil A, Davies PA, Moss SJ, Salituro FG, Hoffmann E, Hammond RS, Robichaud AJ, Quirk MC, Doherty JJ. Preclinical characterization of zuranolone (SAGE-217), a selective neuroactive steroid GABAA receptor positive allosteric modulator. Neuropharmacology. 2020 Dec 15;181:108333. doi: 10.1016/j.neuropharm.2020.108333. Epub 2020 Sep 22. PMID: 32976892; PMCID: PMC8265595.
Clayton AH, Lasser R, Nandy I, Sankoh AJ, Jonas J, Kanes SJ. Zuranolone in Major Depressive Disorder: Results From MOUNTAIN-A Phase 3, Multicenter, Double-Blind, Randomized, Placebo-Controlled Trial. J Clin Psychiatry. 2023a Feb 20;84(2):22m14445. doi: 10.4088/JCP.22m14445. PMID: 36811520.
Clayton AH, Lasser R, Parikh SV, Iosifescu DV, Jung J, Kotecha M, Forrestal F, Jonas J, Kanes SJ, Doherty J. Zuranolone for the Treatment of Adults With Major Depressive Disorder: A Randomized, Placebo-Controlled Phase 3 Trial. Am J Psychiatry. 2023b May 3:appiajp20220459. doi: 10.1176/appi.ajp.20220459. Epub ahead of print. PMID: 37132201.
Deligiannidis KM, Meltzer-Brody S, Gunduz-Bruce H, Doherty J, Jonas J, Li S, Sankoh AJ, Silber C, Campbell AD, Werneburg B, Kanes SJ, Lasser R. Effect of Zuranolone vs Placebo in Postpartum Depression: A Randomized Clinical Trial. JAMA Psychiatry. 2021 Sep 1;78(9):951-959. doi: 10.1001/jamapsychiatry.2021.1559. Erratum in: JAMA Psychiatry. 2022 Jul 1;79(7):740. Erratum in: JAMA Psychiatry. 2023 Feb 1;80(2):191. PMID: 34190962; PMCID: PMC8246337.
Deligiannidis KM, Meltzer-Brody S, Maximos B, Peeper EQ, Freeman M, Lasser R, Bullock A, Kotecha M, Li S, Forrestal F, Rana N, Garcia M, Leclair B, Doherty J. Zuranolone for the Treatment of Postpartum Depression. Am J Psychiatry. 2023 Jul 26:appiajp20220785. doi: 10.1176/appi.ajp.20220785. Epub ahead of print. PMID: 37491938.
Copyright © 2023 to 2025, Simple and Practical Medical Education, LLC. All rights reserved. The content on this website may not be reproduced in any form without express written permission.
Disclaimer: The material on this website is provided as general education for medical professionals. It is not intended or recommended for patients or other laypersons or as a substitute for medical advice, diagnosis, or treatment. Patients must always consult a qualified healthcare professional regarding their diagnosis and treatment. Healthcare professionals should always check this website for the most recently updated information.
Leave a Reply