By Mahendra Dave, MD, and Ronak Dave
Dr. Mahendra Dave is an Associate Clinical Professor of Psychiatry at the Morehouse School of Medicine in Atlanta, Georgia. Mr. Ronak Dave is a medical student at the Medical College of Georgia in Augusta, Georgia.
(This article was last updated on September 28, 2020)
The virus causing COVID-19 is called SARS-CoV-2 which stands for “Severe Acute Respiratory Syndrome associated Coronavirus 2”. The designation “2” is used because this virus is genetically related to, but different from, the SARS-coronavirus which caused the pandemic of 2002-2003, which also originated in China.
On this page, we will present a simple guide for non-specialist medical professionals about the various diagnostic tests used, as of September 2020, to identify either a current or past SARS-CoV-2 infection. There are three main types of tests that we should know about:
1. Molecular (also called the RT-PCR test, SARS-CoV-2 RNA test, or SARS-CoV-2, NAA test)
2. Antigen test
3. Antibody test
It is extremely important to understand that some tests identify CURRENT infection while others identify PAST infection.
Note: Tests in these three categories may be available from multiple manufacturers.
Diagnostic tests to identify current SARS-CoV-2 infection
Who should be tested?
Tests for possible current SARS-CoV-2 infection are recommended not only for persons with symptoms that suggest possible SARS-CoV-2 infection but also for asymptomatic persons because they can transmit the virus as well. Asymptomatic persons in whom testing may be considered include (Centers for Disease Control and Prevention, 2020):
1. Healthcare workers and first responders.
2. Patients in a nursing home
2. A person who came in close contact (less than six feet) of a person with known SARS-CoV-2 infection for 15 minutes or more.
3. A person who lives in a high SARS-CoV-2 transmission zone AND was with a group of more than ten people without all of them wearing masks and staying six feet away from each other.
Note: This is not at all a full discussion of who should be tested for possible SARS-CoV-2 infection.
What are the tests looking for and where?
To identify current infection with the virus, tests look for either the viral RNA2 or viral antigen in the person’s secretions. The sample can be from a nasal swab, oral swab, or saliva.
How long until the results are available?
Some tests can provide results within an hour or less. This is called “point-of-care” testing. In other cases, the sample has to be sent off to a laboratory, so the results take a few days to come back.
As of September 2020, there are two main types of tests that are being used for identifying current infection—Molecular tests and Antigen tests. Let’s briefly look at them one by one.
1. Molecular test
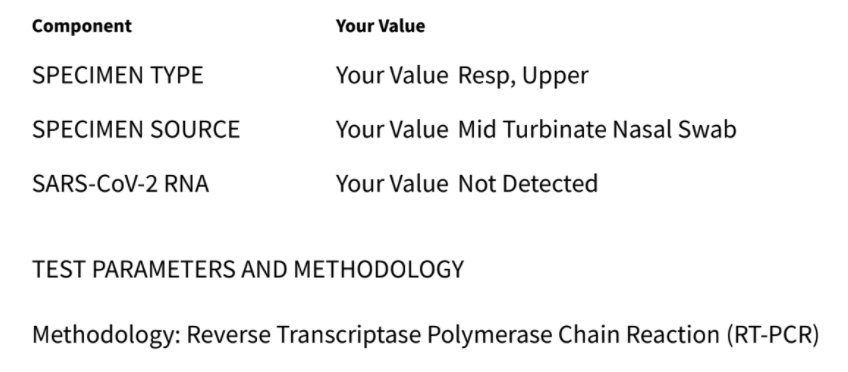
This type of test is based on detecting the amount of SARS-CoV-2 viral RNA in a sample from a nasal swab, throat swab, or (sometimes) saliva. This type of test has several different names:
– Since the test depends on detecting viral RNA, it is also called the “SARS-CoV-2 RNA test.”
– The test is based on a reaction called Reverse Transcription–Polymerase Chain Reaction that is used to amplify the tiny amount of viral RNA and make it detectable. So, it is also called the “RT-PCR test”.
– As the test is based on amplification of the viral nucleic acid (RNA), so it is also called the “SARS-CoV-2, NAA test“, where NAA stands for nucleic acid amplification.
Availability of results: Point-of-care (relatively immediate) or to up to 7 days later.
A description of this test on the Labcorp website is available HERE. and on the Quest Diagnostics website is available HERE.
The images below show two examples of reports from this type of test.
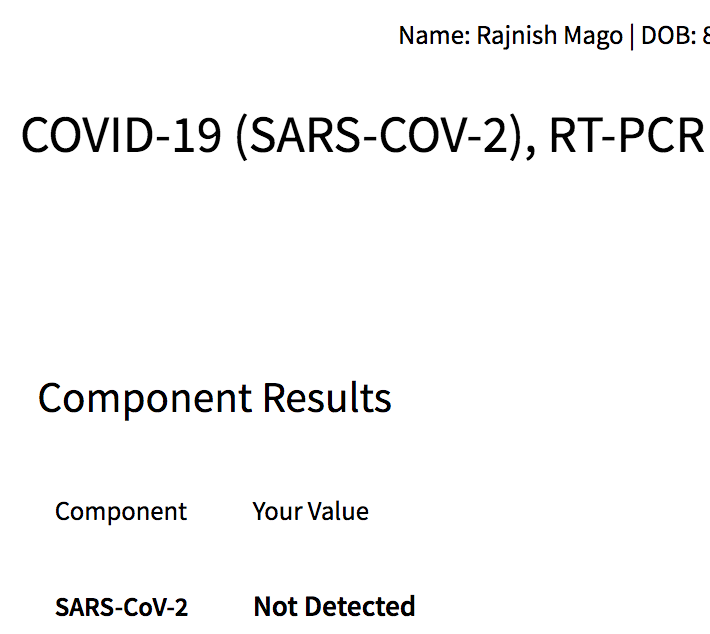
And here is the result of my test:
2. Antigen test
This type of test is based on detecting viral antigen in a sample from a nasal or throat swab.
Antigen tests have two big advantages:
1. They cost less than the molecular tests.
2. They are very specific for the SARS-CoV-2 virus because they detect fragments of proteins on or within the virus.
3. The results are available quickly—in an hour or less.
Antigen tests are often used in point-of-care testing, e.g., at pharmacies.
But, there’s a big problem. The sensitivity of antigen tests for SARS-CoV-2 is not as good as the molecular PCR tests. These tests may become positive only at a higher viral load compared to the molecular or RT-PCR tests discussed above. That is, false-negative results can occur. The bottom line is that if an antigen test is positive, we can assume that the person has SARS-CoV-2 infection. But, if it is negative, a molecular RT-PCR test is needed to rule out a SARS-CoV-2 infection.
Note: Since the antigen test is a point-of-care test, you won’t find it on the Labcorp or Quest Diagnostics websites.
Tests to determine past SARS-CoV-2 infection
It is sometimes useful to know if a person was previously infected with SARS-CoV-2.
Antibody Test
The FDA approved this antibody blood test for the detection of immunoglobulin G (IgG) antibodies to SARS-CoV-2 under an Emergency Use Authorization (August and September 2020).
One reason the antibody test is important is that it helps identify who can donate convalescent plasma, which is being used as a treatment for COVID-19. The idea behind this is that the donor’s antibodies may help fight infection in the person receiving plasma that contains the antibodies.
Here are some important things to keep in mind about the antibody tests:
1. The body takes one to three weeks after the onset of infection to develop these antibodies. So, this test may give false-negative results if the infection occurred only very recently. It is recommended that this test should be done only 14 days ore more after the onset of symptoms.
2. The presence of the antibody does not necessarily mean that the person is immune to future infection with SARS-CoV-2. That issue is not being discussed on this page.
Availability of results: Point-of-care (relatively immediate) or to up to 3 days later.
A description of this test on the Labcorp website is available HERE. and on the Quest Diagnostics website is available HERE.
Related Pages
What mental health clinicians can do about the coronavirus
Warning about using psychotropic medications along with treatments for COVID-19
Could psychotropic medications affect the immune response to coronavirus?
References
Centers for Disease Control and Prevention (CDC). Overview of testing for SARS-CoV-2 (COVID-19). Updated September 18, 2020.
Food and Drug Administration (FDA). Coronavirus testing basics. July 2020.
Mayo Clinic. Coronavirus disease 2019 (COVID-19): Diagnosis and treatment Last accessed September 2020.
Quest Diagnostics. COVID-19 Antibody Test. Accessed 9/9/2020.
World Health Organization (WHO). Technical guidance: Naming the coronavirus disease (COVID-19) and the virus that causes it. Last accessed 9/10/20.
References not used on this page
Dave M, Riesenberg R. Laboratory values in psychiatry clinical trials. ACMR, Atlanta, 2017.
Food and Drug Administration (FDA). Coronavirus (COVID-19) Update: FDA Authorizes First Antigen Test to Help in the Rapid Detection of the Virus that Causes COVID-19 in Patients. May 2020.
Food and Drug Administration (FDA). Investigational COVID-19 Convalescent Plasma. Guidance for Industry. September 2, 2020.
Grifoni E, Valoriani A, Cei F, Lamanna R, Gelli AMG, Ciambotti B, Vannucchi V, Moroni F, Pelagatti L, Tarquini R, Landini G, Vanni S, Masotti L. Interleukin-6 as prognosticator in patients with COVID-19. J Infect. 2020 Sep;81(3):452-482. doi: 10.1016/j.jinf.2020.06.008. Epub 2020 Jun 8. PMID: 32526326; PMCID: PMC7278637.
Huang G, Kovalic AJ, Graber CJ. Prognostic Value of Leukocytosis and Lymphopenia for Coronavirus Disease Severity. Emerg Infect Dis. 2020 Aug;26(8):1839-1841. doi: 10.3201/eid2608.201160. Epub 2020 May 8. PMID: 32384045; PMCID: PMC7392413.
Hundt MA, Deng Y, Ciarleglio MM, Nathanson MH, Lim JK. Abnormal Liver Tests in COVID-19: A Retrospective Observational Cohort Study of 1827 Patients in a Major U.S. Hospital Network. Hepatology. 2020 Jul 29. doi: 10.1002/hep.31487. Epub ahead of print. PMID: 32725890.
Lab Tests Online. D-dimer – Understand the Test. Last modified July 29, 2020.
Paliogiannis P, Mangoni AA, Dettori P, Nasrallah GK, Pintus G, Zinellu A. D-Dimer Concentrations and COVID-19 Severity: A Systematic Review and Meta-Analysis. Front Public Health. 2020 Aug 4;8:432. doi: 10.3389/fpubh.2020.00432. PMID: 32903841; PMCID: PMC7438945.
Poggiali E, Zaino D, Immovilli P, Rovero L, Losi G, Dacrema A, Nuccetelli M, Vadacca GB, Guidetti D, Vercelli A, Magnacavallo A, Bernardini S, Terracciano C. Lactate dehydrogenase and C-reactive protein as predictors of respiratory failure in CoVID-19 patients. Clin Chim Acta. 2020 Oct;509:135-138. doi: 10.1016/j.cca.2020.06.012. Epub 2020 Jun 9. PMID: 32531257; PMCID: PMC7282743.
Tavakolpour S, Rakhshandehroo T, Wei EX, Rashidian M. Lymphopenia during the COVID-19 infection: What it shows and what can be learned. Immunol Lett. 2020 Sep;225:31-32. doi: 10.1016/j.imlet.2020.06.013. Epub 2020 Jun 20. PMID: 32569607; PMCID: PMC7305732.
Vargas-Vargas M, Cortés-Rojo C. Ferritin levels and COVID-19. Rev Panam Salud Publica. 2020 Jun 1;44:e72. doi: 10.26633/RPSP.2020.72. PMID: 32547616; PMCID: PMC7286435.
Copyright © 2020, Simple and Practical Medical Education, LLC. All rights reserved. May not be reproduced in any form without express written permission.
Disclaimer: The content on this website is provided as general education for medical professionals. It is not intended or recommended for patients or other laypersons or as a substitute for medical advice, diagnosis, or treatment. Patients must always consult a qualified health care professional regarding their diagnosis and treatment. Healthcare professionals should always check this website for the most recently updated information.


Thank you for creating this easy-to-understand guide on the various tests. I appreciate it!