This article was first published on November 7, 2015. It was last reviewed, updated, or edited on August 24, 2023.
Clozapine (US brand names Clozaril®, Fazaclo® ODT, Versacloz®, and generic) is a second-generation (“atypical”) antipsychotic.
On this page, we present some basic information about this medication. Other articles on this website with more advanced information and tips related to this medication are linked to under Related Pages below.
FDA-approved indications
1. Treatment-resistant schizophrenia
2. Reducing suicidal behavior in patients with schizophrenia or schizoaffective disorder
Mechanism of Action/ Pharmacodynamics
Clozapine is an antagonist at dopamine D2 and the serotonin 2A (5-HT2A) receptors.
It is also an antagonist at:
– All the other dopaminergic receptors D1 through D5.
– Some other serotonergic receptors: 5-HT1A, 5-HT2C, 5-HT3, 5-HT6, and 5-HT7.
– Adrenergic alpha-1A, cholinergic M1, and histaminergic H1 receptors.
Pharmacokinetics
Metabolism
Clozapine is metabolized by cytochrome P450 1A2, 2D6, and 3A4.
Half-life
At steady state, the mean elimination half-life of clozapine is about 12 hours, but with a quite wide range from 4 to 66 hours (Prescribing Information).
Boxed warning
There are 5 items in the boxed warning in the Prescribing Information. Here is the entire text of the boxed warning :
1. AGRANULOCYTOSIS
BECAUSE OF A SIGNIFICANT RISK OF AGRANULOCYTOSIS, A POTENTIALLY LIFE-THREATENING ADVERSE EVENT, CLOZARIL® (CLOZAPINE) SHOULD BE RESERVED FOR USE IN (1) THE TREATMENT OF SEVERELY ILL PATIENTS WITH SCHIZOPHRENIA WHO FAIL TO SHOW AN ACCEPTABLE RESPONSE TO ADEQUATE COURSES OF STANDARD ANTIPSYCHOTIC DRUG TREATMENT, OR (2) FOR REDUCING THE RISK OF RECURRENT SUICIDAL BEHAVIOR IN PATIENTS WITH SCHIZOPHRENIA OR SCHIZOAFFECTIVE DISORDER WHO ARE JUDGED TO BE AT RISK OF REEXPERIENCING SUICIDAL BEHAVIOR.
PATIENTS BEING TREATED WITH CLOZAPINE MUST HAVE A BASELINE WHITE BLOOD CELL (WBC) COUNT AND ABSOLUTE NEUTROPHIL COUNT (ANC) BEFORE INITIATION OF TREATMENT AS WELL AS REGULAR WBC COUNTS AND ANCs DURING TREATMENT AND FOR AT LEAST 4 WEEKS AFTER DISCONTINUATION OF TREATMENT (SEE WARNINGS).
CLOZAPINE IS AVAILABLE ONLY THROUGH A DISTRIBUTION SYSTEM THAT ENSURES MONITORING OF WBC COUNT AND ANC ACCORDING TO THE SCHEDULE DESCRIBED BELOW PRIOR TO DELIVERY OF THE NEXT SUPPLY OF MEDICATION (SEE WARNINGS).
2. SEIZURES
SEIZURES HAVE BEEN ASSOCIATED WITH THE USE OF CLOZAPINE. DOSE APPEARS TO BE AN IMPORTANT PREDICTOR OF SEIZURE, WITH A GREATER LIKELIHOOD AT HIGHER CLOZAPINE DOSES. CAUTION SHOULD BE USED WHEN ADMINISTERING CLOZAPINE TO PATIENTS HAVING A HISTORY OF SEIZURES OR OTHER PREDISPOSING FACTORS. PATIENTS SHOULD BE ADVISED NOT TO ENGAGE IN ANY ACTIVITY WHERE SUDDEN LOSS OF CONSCIOUSNESS COULD CAUSE SERIOUS RISK TO THEMSELVES OR OTHERS. (SEE WARNINGS.)
3. MYOCARDITIS
ANALYSES OF POSTMARKETING SAFETY DATABASES SUGGEST THAT CLOZAPINE IS ASSOCIATED WITH AN INCREASED RISK OF FATAL MYOCARDITIS, ESPECIALLY DURING, BUT NOT LIMITED TO, THE FIRST MONTH OF THERAPY. IN PATIENTS IN WHOM MYOCARDITIS IS SUSPECTED, CLOZAPINE TREATMENT SHOULD BE PROMPTLY DISCONTINUED. (SEE WARNINGS.)
4. OTHER ADVERSE CARDIOVASCULAR AND RESPIRATORY EFFECTS
ORTHOSTATIC HYPOTENSION, WITH OR WITHOUT SYNCOPE, CAN OCCUR WITH CLOZAPINE TREATMENT. RARELY, COLLAPSE CAN BE PROFOUND AND BE ACCOMPANIED BY RESPIRATORY AND/OR CARDIAC ARREST. ORTHOSTATIC HYPOTENSION IS MORE LIKELY TO OCCUR DURING INITIAL TITRATION IN ASSOCIATION WITH RAPID DOSE ESCALATION. IN PATIENTS WHO HAVE HAD EVEN A BRIEF INTERVAL OFF CLOZAPINE, i.e., 2 OR MORE DAYS SINCE THE LAST DOSE, TREATMENT SHOULD BE STARTED WITH 12.5 mg ONCE OR TWICE DAILY. (SEE WARNINGS and DOSAGE AND ADMINISTRATION.)
SINCE COLLAPSE, RESPIRATORY ARREST AND CARDIAC ARREST DURING INITIAL TREATMENT HAS OCCURRED IN PATIENTS WHO WERE BEING ADMINISTERED BENZODIAZEPINES OR OTHER PSYCHOTROPIC DRUGS, CAUTION IS ADVISED WHEN CLOZAPINE IS INITIATED IN PATIENTS TAKING A BENZODIAZEPINE OR ANY OTHER PSYCHOTROPIC DRUG. (SEE WARNINGS.)
5. INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS
ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS TREATED WITH ANTIPSYCHOTIC DRUGS ARE AT AN INCREASED RISK OF DEATH. ANALYSES OF SEVENTEEN PLACEBO-CONTROLLED TRIALS (MODAL DURATION OF 10 WEEKS), LARGELY IN PATIENTS TAKING ATYPICAL ANTIPSYCHOTIC DRUGS, REVEALED A RISK OF DEATH IN THE DRUG-TREATED PATIENTS OF BETWEEN 1.6 TO 1.7 TIMES THE RISK OF DEATH IN PLACEBO-TREATED PATIENTS. OVER THE COURSE OF A TYPICAL 10-WEEK CONTROLLED TRIAL, THE RATE OF DEATH IN DRUG-TREATED PATIENTS WAS ABOUT 4.5%, COMPARED TO A RATE OF ABOUT 2.6% IN THE PLACEBO GROUP. ALTHOUGH THE CAUSES OF DEATH WERE VARIED, MOST OF THE DEATHS APPEARED TO BE EITHER CARDIOVASCULAR (e.g., HEART FAILURE, SUDDEN DEATH) OR INFECTIOUS (e.g., PNEUMONIA) IN NATURE. OBSERVATIONAL STUDIES SUGGEST THAT, SIMILAR TO ATYPICAL ANTIPSYCHOTIC DRUGS, TREATMENT WITH CONVENTIONAL ANTIPSYCHOTIC DRUGS MAY INCREASE MORTALITY. THE EXTENT TO WHICH THE FINDINGS OF INCREASED MORTALITY IN OBSERVATIONAL STUDIES MAY BE ATTRIBUTED TO THE ANTIPSYCHOTIC DRUG AS OPPOSED TO SOME CHARACTERISTIC(S) OF THE PATIENTS IS NOT CLEAR. CLOZARIL® (CLOZAPINE) IS NOT APPROVED FOR THE TREATMENT OF PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS (see WARNINGS).for clozapine.
Warnings and Precautions
1. Gastrointestinal hypomotility may occur with the use of clozapine which may be associated with constipation and in severe cases with intestinal obstruction, fecal impaction, megacolon, and intestinal ischemia or infarction.
Recommendation: Close monitoring and treatment are recommended if constipation develops in a patient.
2. Eosinophilia (eosinophil count of >700/µL) with organ involvement is seen in some cases which may lead to myocarditis, pancreatitis, hepatitis, colitis, and nephritis.
Recommendation: Discontinue treatment if there is organ involvement due to eosinophilia.
3. QT prolongation may occur with clozapine treatment.
Recommendations: Before starting treatment with clozapine, a careful history and physical examination, and a baseline ECG are recommended.
If symptoms of torsades de pointes or other arrhythmias develop, immediate cardiac evaluation and discontinuation of clozapine are recommended.
4. Baseline evaluation (weight, fasting blood sugar, and lipid panel) may be needed before starting clozapine. And, monitoring of metabolic changes such as hyperglycemia, dyslipidemia, and weight gain is recommended.
5. It is recommended to monitor for hepatotoxicity as it can be fatal in some cases.
6. If neuroleptic malignant syndrome (NMS) develops, discontinue treatment.
7. If a fever develops, immediately evaluate for an infection and for neutropenia.
8. Avoid concomitant use with anticholinergic drugs. Be cautious while prescribing in patients with prior history of constipation, urinary retention, benign prostatic hypertrophy, and other conditions that may worsen with anticholinergic treatment.
9. Advise caution while operating machinery or driving.
Potential side effects
Please see THIS PAGE for a handout listing both the common and less common side effects of this medication along with the percentages of patients who report them.
Potential drug interactions
1. Reduce clozapine dose to 1/3rd when using concomitantly with strong CYP1A2 inhibitors such as fluvoxamine, ciprofloxacin, enoxacin, etc.
2. It is not recommended to use clozapine with strong CYP3A4 inducers (carbamazepine, phenytoin, St. John’s wort, and rifampin).
3. Clozapine dose reduction is recommended when CYP1A2 inducers (tobacco smoke) or CYP3A4 inducers are discontinued.
4. Anticholinergic toxicity may occur if clozapine is used concomitantly with anticholinergic drugs.
Dosage
Starting: 12.5 mg once or twice daily, with or without food
Titration: Increase the total daily dosage in increments of 25 mg to 50 mg per day
Target: 300 – 450 mg per day, in divided doses, by the end of 2 weeks
Subsequent increases: Increments of 100 mg or less, once or twice weekly
Maximum: 900 mg daily
Important instructions
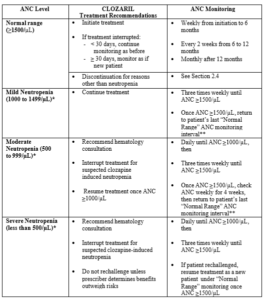
1. Clozapine can cause severe neutropenia. As a result, clozapine is available only through a restricted program under a Risk Evaluation Mitigation Strategy (REMS) called the Clozapine REMS Program (see Related Pages below). Prior to initiating treatment with clozapine, a baseline ANC must be obtained. The baseline ANC must be at least 1500/µL for the general population, and at least 1000/µL for patients with documented Benign Ethnic Neutropenia (BEN). To continue treatment, the ANC must be monitored regularly.
Clozaril Treatment Recommendations Based on Absolute Neutrophil Count (ANC) Monitoring for the General Patient Population
* Confirm all initial reports of ANC less than 1500/µL with a repeat ANC measurement within 24 hours
** If clinically appropriate
2. Dose adjustments may be necessary in patients with concomitant use of:
Strong CYP1A2 inhibitors (e.g., fluvoxamine, ciprofloxacin, or enoxacin): Use 1/3rd dose of clozapine
Moderate or weak CYP1A2 inhibitors (e.g., oral contraceptives, or caffeine): Monitor for side effects and consider reducing the dose of clozapine if necessary.
CYP2D6 or CYP3A4 inhibitors (e.g., cimetidine, escitalopram, erythromycin, paroxetine, bupropion, fluoxetine, quinidine, duloxetine, terbinafine, or sertraline); Monitor for side effects and consider reducing the dose of clozapine if necessary.
Strong CYP3A4 inducers (carbamazepine, phenytoin, St. John’s wort, and rifampin): Concomitant use of clozapine is not recommended. If necessary, consider increasing the clozapine dose. Also, consider reducing the clozapine dosage when discontinuing coadministered enzyme inducers.
Moderate or weak CYP1A2 (tobacco smoking) or CYP3A4 inducers: Monitor for decreased effectiveness. Consider increasing the clozapine dose if necessary.
3. Seizures have occurred with clozapine treatment. The risk is dose-related. Use caution when administering clozapine to patients with a history of seizures or other predisposing risk factors for seizure.
Dosage forms and strengths
Oral tablet (Clozaril® and generic): 25 mg (scored) and 100 mg (scored). Generic also available in 12.5 mg, 50 mg, and 200 mg strengths.
Orally-disintegrating tablet (Fazaclo® ODT and generic): 12.5 mg, 25 mg, 100 mg, 150 mg, and 200 mg
Oral suspension (Versacloz®): 50 mg/mL
Important! This page does not provide all the information needed to prescribe this medication. Please refer to the full Prescribing Information (see link below) before prescribing this medication.
Related Pages
Key information about seizures with clozapine
Clozapine for schizophrenia in children?
Changes in rules about clozapine
Are the clozapine-norclozapine ratio and norclozapine level useful?
How to check and interpret clozapine levels
Can oral contraceptives affect clozapine levels?
Clozapine-induced hypersalivation (sialorrhea)
Clozapine-induced hypersalivation (sialorrhea): Treatment
Benign ethnic neutropenia (BEN)
Caffeine can significantly change serum clozapine levels
Potential side effects of clozapine (Clozaril®) handout
References
Clozapine (Clozaril®) Prescribing information
Clozapine (Fazaclo® ODT) Prescribing information
Clozapine (Versacloz®) Prescribing Information
Copyright © 2015 to 2025, Simple and Practical Medical Education, LLC. All rights reserved. May not be reproduced in any form without express written permission.
Disclaimer: The content on this website is provided as general education for medical professionals. It is not intended or recommended for patients or other laypersons or as a substitute for medical advice, diagnosis, or treatment. Patients must always consult a qualified healthcare professional regarding their diagnosis and treatment. Healthcare professionals should always check this website for the most recently updated information.
Leave a Reply